Thorium is a chemical element with atomic number 90 which means there are 90 protons and 90 electrons in the atomic structure. The chemical symbol for Thorium is Th. Thorium metal is silvery and tarnishes black when exposed to air, forming the dioxide.
It provides atomic mass, mass excess, nuclear binding energy, nucleon separation energies, Q-values, and nucleon residual interaction parameters for atomic nuclei of the isotope Th-261 (Thorium, atomic number Z = 90, mass number A = 261). Thorium is a chemical element with atomic number 90 which means there are 90 protons in its nucleus. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol Z. Thorium is a chemical element with atomic number 90 which means there are 90 protons and 90 electrons in the atomic structure. The chemical symbol for Thorium is Th. Atomic Mass of Thorium Atomic mass of Thorium is 232.0381 u.
Element With Atomic Number 17
Learn about this topic in these articles:
Assorted References
- fissile material
- In fissile material
the fertile materials uranium-238 and thorium-232, respectively. A fertile material, not itself capable of undergoing fission with low-energy neutrons, is one that decays into fissile material after neutron absorption within a reactor. Thorium-232 and uranium-238 are the only two naturally occurring fertile materials.
Read More
- In fissile material
- half-life
- In thorium
…isotopes, predominantly the very long-lived thorium-232 (1.40 × 1010-year half-life), the parent of the thorium radioactive decay series. Other isotopes occur naturally in the uranium and actinium decay series, and thorium is present in all uranium ores. Thorium-232 is useful in breeder reactors because on capturing slow-moving neutrons
Read More
- In thorium
- helium dating
- In helium dating
isotopes uranium-235, uranium-238, and thorium-232. Because of this decay, the helium content of any mineral or rock capable of retaining helium will increase during the lifetime of that mineral or rock, and the ratio of helium to its radioactive progenitors then becomes a measure of geologic time. If the…
Read More
- In helium dating
- occurrences
- In thorium processing
…in nature is the isotope thorium-232 (several other isotopes exist in trace amounts or can be produced synthetically). This slightly radioactive material is not fissile itself, but it can be transformed in a nuclear reactor to the fissile uranium-233. Since thorium is present in the Earth’s crust in about three…
Read More
- In thorium processing
- thermonuclear warhead design
- In thermonuclear warhead: Enhanced designs
Uranium-238 and thorium-232 (and some other fissionable materials) cannot maintain a self-sustaining fission explosion, but these isotopes can be made to fission by an externally maintained supply of fast neutrons from fission or fusion reactions. Thus, the yield of a nuclear weapon can be increased by surrounding…
Read More
- In thermonuclear warhead: Enhanced designs
- uranium-thorium-lead dating
- In uranium-thorium-lead dating
…uranium-238 and the thorium isotope thorium-232.
Read More
- In uranium-thorium-lead dating
production of

Thorium Atomic Number And Mass
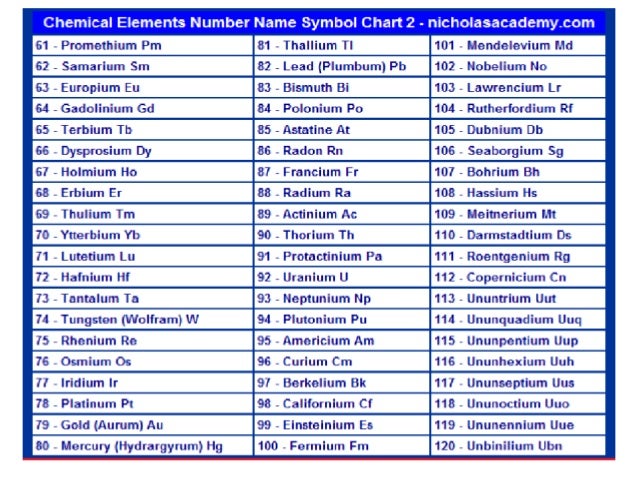
Atomic Number Chart
- radioactive heat
- In rock: Radioactive heat generation
…important are uranium-235, uranium-238, and thorium-232—and a few with a lower atomic number, such as potassium-40.
Read More
- In rock: Radioactive heat generation
- uranium-233
- In nuclear reactor: Fissile and fertile materials
When a nucleus of thorium-232 absorbs, or “captures,” a neutron, it becomes thorium-233, whose half-life is approximately 21.83 minutes. After that time the nuclide decays through electron emission to protactinium-233, whose half-life is 26.967 days. The protactinium-233 nuclide in turn decays through electron emission to yield uranium-233.
Read More
- In nuclear reactor: Fissile and fertile materials
