Amedeo Avogadro was an Italian scientist who formulated what is now known as Avogadro's law. Hailed as a founder of the atomic-molecular theory, he was the first scientist to realize that elements could exist in the form of molecules rather than as individual atoms. Contrary to the beliefs of generations of chemistry students, Avogadro’s number—the number of particles in a unit known as a mole—was not discovered by Amadeo Avogadro (1776-1856).
07th May 2019 @ 12 min read
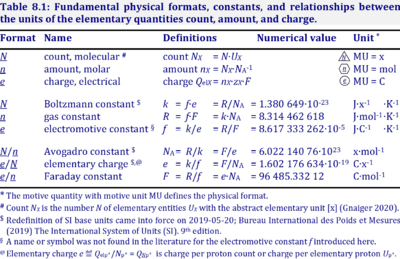
The Avogadro constant or (the Avogadro number earlier) is the number of elementary units in one mole of any substance. The Avogadro constant is denoted as NA. It has the dimension of the reciprocal amount of substance (mol−1). The approximate value of NA is 6.022 × 1023 mol−1. This means one mole of any substance contains 6.022 × 1023 elementary particles. The Avogadro constant is named after Italian scientist Amedeo Avogadro.
These elementary units in one mole can be anything like atoms, molecules, ions, electrons, protons, neutrons, particles of sand. So, when we say one mole of sodium chloride, it means 6.022 × 1023 molecules of sodium chloride.
Values of Avogadro's Constant
The value of the Avogadro constant is revised over a period of time. As of the 2019 redefinition of the SI base units, the value of the Avogadro constant is fixed to 6.02214076×1023mol−1. This is the exact value of the constant. The table below mentions the value of the constant in different units.
| Value | Unit |
|---|---|
| 6.022 140 76 × 1023 | mol−1 |
| 6.022 140 76 × 1026 | kmol−1 |
| 2.731 597 099 802 001 2 × 1026 | lb-mol−1 |
| 1.707 248 187 376 250 75 × 1025 | oz-mol−1 |
| 0.602 214 076 | mL mol−1 Å |
History of Avogadro's Constant
The Avogadro constant has a long history. The constant is named in honour of Avogadro, but he did not discover it. In 1811, Avogadro discovered the relationship between the volume of gas and the amount of gas through his experiments. He was the first to proposed the volume of a gas is directly proportional to the amount of the gas at constant pressure and temperature. This is today we call Avogadro's law or Avogadro's hypothesis. His work does not mention Avogadro's constant.
Perrin's Work
French Nobel Laureate Jean Baptiste Perrin estimated the Avogadro number with several methods. And he credited the naming of the number to Avogadro in 1909. Perrin named the number Avogadro's number, not Avogadro's constant. This name had continued till 1971. In 1971, the International System of Unit (SI) introduced a new quantity called Avogadro's constant. The Avogadro constant has the same numerical value as the Avogadro number, but they differ in the unit which will be explained later in this article.
Perrin defined the Avogadro number as the number of atoms in one gram of hydrogen (one gram-molecule). This definition was later revised to the number of atoms in 12 grams of carbon-12 (12C).
Loschmidt's Estimation
Before Perrin, Loschmidt also made a significant contribution to the number. Josef Loschmidt was an Austrian scientist who is notable for his work on estimation of the diameter of the molecules in the air. Through his method, it is possible to calculate the number density (the number of molecules or atoms per unit volume). This quantity is closely relative to the Avogadro constant. The relationship between them is discussed later in this article. The number density of an ideal gas is called as the Loschmidt constant. In many of German literature, these two constants are interchangeable. They can easily be distinguished from their units. The Avogadro constant is also denotated as L in honour of Loschmidt.
Other Efforts
Robert Millikan was an American physicist and Nobel Laureate. He successfully measured the charge on an electron in 1910. The electric charge per mole (the Faraday constant) of electrons had already known at that time. With the help of these two quantities, the electric charge on an electron and the Faraday constant, it is possible to calculate the number of electrons per mole. The value of this number of electrons per mole is the same as Avogadro's constant.
One of a modern method to estimate the value of the constant is X-ray crystallography. This method estimates the constant by determining the number of silicon atoms in a crystal cell, the volume per unit cell, and the molar volume.
The measurement of the accurate value of the Avogadro's constant is always troublesome. Over the period, the new methods were developed, and the Avogadro constant has continuously been improvised. From 2019, the international committee fixed the value of the Avogadro's constant exactly to 6.022 140 76 × 1023 mol−1.
2019 Redefinition and Prior Definition of Avogadro's Constant
As discussed above, the 2019 redefinition of the Avogadro constant is 6.022 140 76 × 1023 mol−1. The consequence of this redefinition is the prior definition of the constant is no longer valid. Before the 2019 redefinition, the value of the constant was defined as the amount of atoms presents in 12g of carbon-12 (12C). Also, because of the definition the molar mass constant (Mu) is no longer exactly equal to 1 g mol−1. Instead, it is approximately equal to 1 g mol−1. This is summarised in the table below.
| 2019 Redefinition | Prior to 2019 Redefinition |
|---|---|
| NA = 6.022 140 76 × 1023 mol−1 | The value of NA is the number of 12C atoms in 12 g of carbon-12. |
| The molar mass constant is approximately equal to 1 g mol−1 (Mu ≈ 1 g mol−1). | Mu is exactly equal to 1 g mol−1 (Mu = 1 g mol−1). |

Note: The difference in the value of the Avogadro constant before and after the 2019 definition is very small. The redefinition would not affect most of the calculations unless the high degree of precision is needed. For practical calculations, we can take NA = 6.022 × 1023 mol−1.
Avogadro's Constant and Mole
The Avogadro constant and the mole are related quantities. In fact, the Avogadro constant is defined in terms of the mole. The value of Avogadro's constant is the number of elementary units in one mole of any substance. The definition is universally true. The below equation establishes the relation between both.
Avogadro's Constant and Molar Mass
We can use the Avogadro constant to determine the mass of any atom if we know the molar mass of that atom. This statement is also true for molecules. The molar mass is the mass of one mole of a given sample. It is expressed in g mol−1. The relation between both is as follows:
where mi is the mass of atom i and Mi is molar mass of atom i.
Avogadro's Constant and Avogadro's Number
The Avogadro constant and the Avogadro number have the same numerical value. They only differ in the unit. The Avogadro number is a dimensionless quantity, but the Avogadro constant has the dimension of the reciprocal amount of substance (mol−1). The below table describes the same.
Avogadro Full Name

| Avogadro's Constant | Avogadro's Number |
|---|---|
| The constant has the unit of mol−1. | It is a dimensionless quantity. |
| It is denoted as NA. | We use N to denote the Avogadro number. |
| NA = 6.022 × 1023 mol−1 | NA = 6.022 × 1023 |
Avogadro's Constant and Boltzmann's constant
The Boltzmann constant is an important physical constant which plays a vital role in classical statistical mechanics. It is denoted as kB or simply k. The Avogadro constant is related to the Boltzmann constant by the gas constant R.
Avogadro's Constant and Loschmidt's Constant
The Loschmidt constant is the number density (the number of molecules per unit volume). For an ideal gas, the relationship between the Loschmidt constant and the Avogadro constant at STP (P0 = 1 atm, T0 = 273.15 K) is described in the equation below.
Avogadro's Constant and Faraday's Constant
The Faraday constant (F) is the Avogadro constant times the elementary charge (e).
Avogadro's Constant and Unified Mass Unit
The unified mass unit or the dalton (u) is the ratio of the molar mass constant (Mu) and the Avogadro constant.
where mu is the atomic mass constant.
The value precise value of Mu is 0.999 999 999 65(30) g mol−1. But for practical purposes, we can say Mu ≈ 1 g mol−1.
Examples
Example 1: To Determine Calcium Atoms
Statement: For 100 g of calcium in a beaker, calculate the number of calcium atoms in the beaker?
Solution: The molecular weight of calcium is 40.1 g mol−1. The number of moles of calcium in the beaker is
The number of calcium atoms in the beaker is calculated as:
Therefore, the number of calcium atoms is 1.50 × 1024.
Example 2: To Determine Total Molecules in Sodium Chlorine Solution
Statement: Consider 50.0 g of NaCl is dissolved in 200 g of water. Estimate the total molecules in the solution?
Solution: The molecular weight of NaCl and water is 58.44 g mol−1 and 18.01 g mol−1.

The moles of NaCl in 50.0 g:
The moles of H2O in 100 g:
When 1 mol of NaCl dissociates, 1 mol of Na+ and 1 mol of Cl− are formed. So, when 0.855 5 mol of NaCl dissociates, 0.855 5 mol of Na+ and 0.855 5 mol of Cl− are formed.
$underset{1,text{mol}}{ce{Na+}}$ + $underset{1,text{mol}}{ce{Cl-}}$}' alt='>Thus, the total number of moles after the dissociation is the sum of the moles of Na+, Cl−, and H2O.
The total number of molecules in the solution is
Therefore, the total number of moles in NaCl solution is 4.374 × 1024 mol.
Amedeo Avogadro Full Name
Example 3: To Determine Mass of Sodium Atom
Statement: The molar mass of sodium-23 is 22.989 g mol−1. Calculates the mass of a sodium atom?
Solution: Let mNa and MNa be the atomic mass and molar mass of sodium-23. Thus, MNa = 22.989 g mol−1.
Now, mNa can be determined using the formula below.
Therefore, the mass of a sodium-23 atom is 3.817 × 10−23 g.
Example 4: To Determine Molecular Mass of Iodine gas
Statement: The atomic mass of iodine is 126.9 g mol−1. Determine the molecular mass of iodine gas?
Amedeo Avogadro Full Name Pronounce
Solution: The iodine gas is a diatomic gas. The molecular formula is I2. So, the molar mass of I2 is twice the molar mass of I.
Now, mI2 can be determined as:
Therefore, the mass of a iodine molecule is 4.208 × 10−22 g.
Associated Articles
If you appreciate our work, consider supporting us on ❤️ patreon.- 4
- cite
- response
Copy Article Cite
29th Oct 2019
In 1811 Avogadro put forward a hypothesis that was neglected by his contemporaries for years. Eventually proven correct, this hypothesis became known as Avogadro’s law, a fundamental law of gases.
The contributions of the Italian chemist Amedeo Avogadro (1776–1856) relate to the work of two of his contemporaries, Joseph Louis Gay-Lussac and John Dalton. Gay-Lussac’s law of combining volumes (1808) stated that when two gases react, the volumes of the reactants and products—if gases—are in whole number ratios. This law tended to support Dalton’s atomic theory, but Dalton rejected Gay-Lussac’s work. Avogadro, however, saw it as the key to a better understanding of molecular constituency.
Avogadro’s Hypothesis
In 1811 Avogadro hypothesized that equal volumes of gases at the same temperature and pressure contain equal numbers of molecules. From this hypothesis it followed that relative molecular weights of any two gases are the same as the ratio of the densities of the two gases under the same conditions of temperature and pressure. Avogadro also astutely reasoned that simple gases were not formed of solitary atoms but were instead compound molecules of two or more atoms. (Avogadro did not actually use the word atom; at the time the words atom and molecule were used almost interchangeably. He talked about three kinds of “molecules,” including an “elementary molecule”—what we would call an atom.) Thus Avogadro was able to overcome the difficulty that Dalton and others had encountered when Gay-Lussac reported that above 100°C the volume of water vapor was twice the volume of the oxygen used to form it. According to Avogadro, the molecule of oxygen had split into two atoms in the course of forming water vapor.
bio-avogadro.jpg
Edgar Fahs Smith Collection, Kislak Center for Special Collections, Rare Books and Manuscripts, University of Pennsylvania
Curiously, Avogadro’s hypothesis was neglected for half a century after it was first published. Many reasons for this neglect have been cited, including some theoretical problems, such as Jöns Jakob Berzelius’s “dualism,” which asserted that compounds are held together by the attraction of positive and negative electrical charges, making it inconceivable that a molecule composed of two electrically similar atoms—as in oxygen—could exist. In addition, Avogadro was not part of an active community of chemists: the Italy of his day was far from the centers of chemistry in France, Germany, England, and Sweden, where Berzelius was based.
Personal Life
Avogadro was a native of Turin, where his father, Count Filippo Avogadro, was a lawyer and government leader in the Piedmont (Italy was then still divided into independent countries). Avogadro succeeded to his father’s title, earned degrees in law, and began to practice as an ecclesiastical lawyer. After obtaining his formal degrees, he took private lessons in mathematics and sciences, including chemistry. For much of his career as a chemist he held the chair of physical chemistry at the University of Turin.
The information contained in this biography was last updated on November 30, 2017.
